In 1999, General Electric (GE) announced the development of a high-pressure/high-temperature (HPHT) treatment to improve the color of type II diamonds from brownish to almost colorless. Although the process was restricted to a limited percentage of diamonds, the discovery threw the market into a panic; almost simultaneously the gemological community was activated to develop a method for the identification of the treatment and it was immediately clear that the task was not so easy to accomplish. The standard methods did not provide for any useful information: all the advanced techniques were put at work and the most promising was the photoluminescence spectroscopy. Since then this methodology of investigation turns out to be the architrave on which the most widespread protocols of investigation on diamonds are grounding in gemological laboratories all over the world.
Photoluminescence
The definition of photoluminescence (PL) used in gemology is slightly different from the one used in the scientific field of physics in general. “Our” conception, in fact, refers exclusively to the emission of radiation from a gem when stimulated by a source in the visible or infrared field. If ultraviolet emission is used, we call this reaction “fluorescence” or “phosphorescence” if it remains persistent for a period of time following the switching off of the light source. Since it was originally developed for the study of diamonds, the term “laser” for the source and “typically in low temperature conditions” for the methodology of analysis were also included in the gemological definition. It is in fact known that the emission peaks due to active optical defects useful in diamonds analysis are identifiable with greater accuracy in low temperature conditions. However, even if originally limited to the diamond field, the use of this technique is having major developments every day even in the study of color stones where the cryogenic technique can not usually be used. Diamond, in fact, has characteristics of resistance to fast changes in temperature that almost all the color gems do not possess.
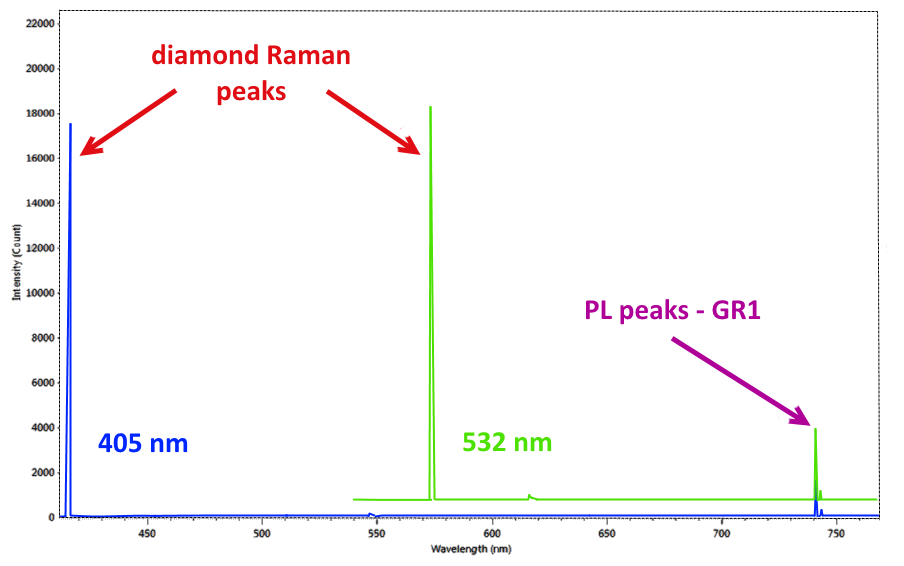
The PL spectrometer
It has been proved laser sources are the most efficient ones in order to stimulate PL emissions; for this reason PL spectrometers generally share the same configuration with the Raman ones. In many cases, in fact, a Raman spectrometer can also work as a PL spectrometer. Another requirement the two techniques have in common is the extreme sensitivity required in the spectrometers used. If the Raman signal, by its very definition, is caused by the inelastic scattering of 1 photon in 10 million, so extremely weak, the “defects” in the atomic structure of the diamonds that generate PL emission peaks are also of minimal entity (up to less than 10 parts per billion). While using the same tool, the two techniques present however a substantial difference. While Raman scattering is constant regardless of the source used, and therefore its identification is performed by means of a “relative” scale, expressed in cm-1 where the 0 corresponds to the laser wavelength of the instrument, the PL peaks are emitted at constant energy for a given material, so their identification is made on an absolute scale expressed in nm (Figure 1). Considering the high sensitivity and resolution requirements necessary for the study of diamonds, the most advanced modern PL systems involve the use of multiple laser sources of different wavelengths and the adoption of spectrometers with resolutions of few tenths of nm (Figure 2).
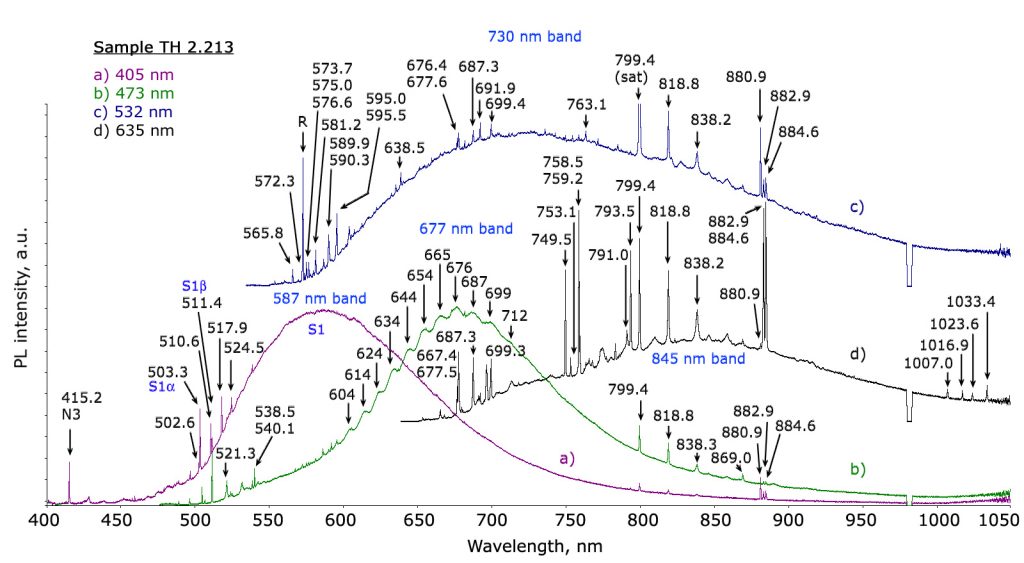
Gemological applications: diamonds
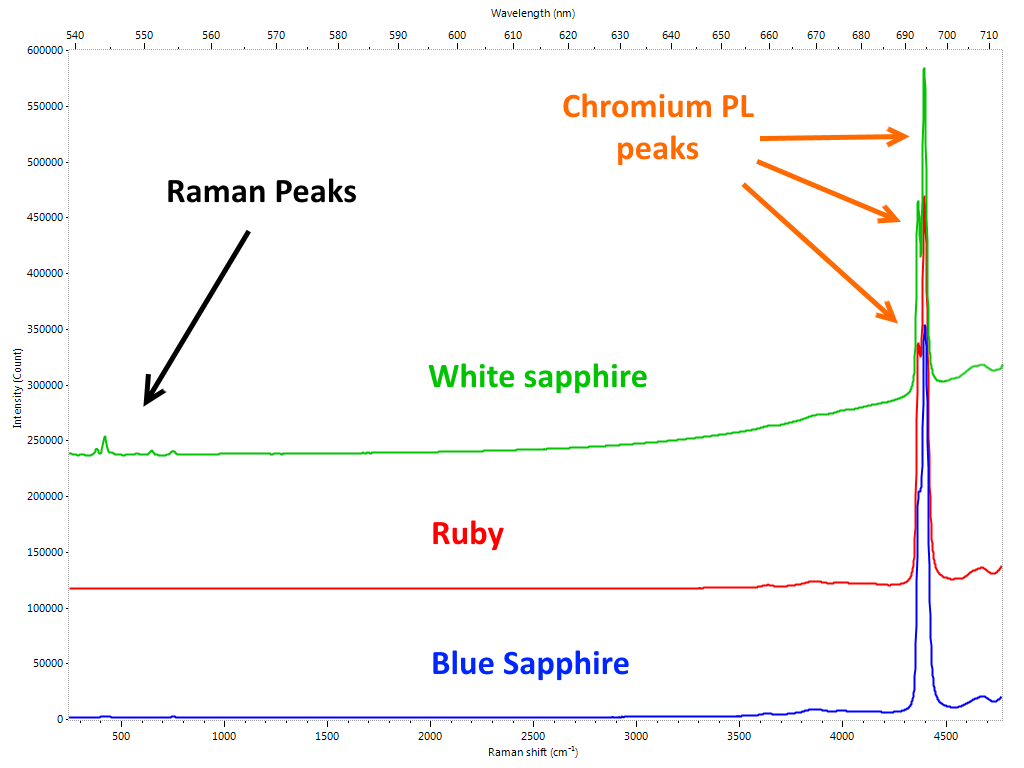
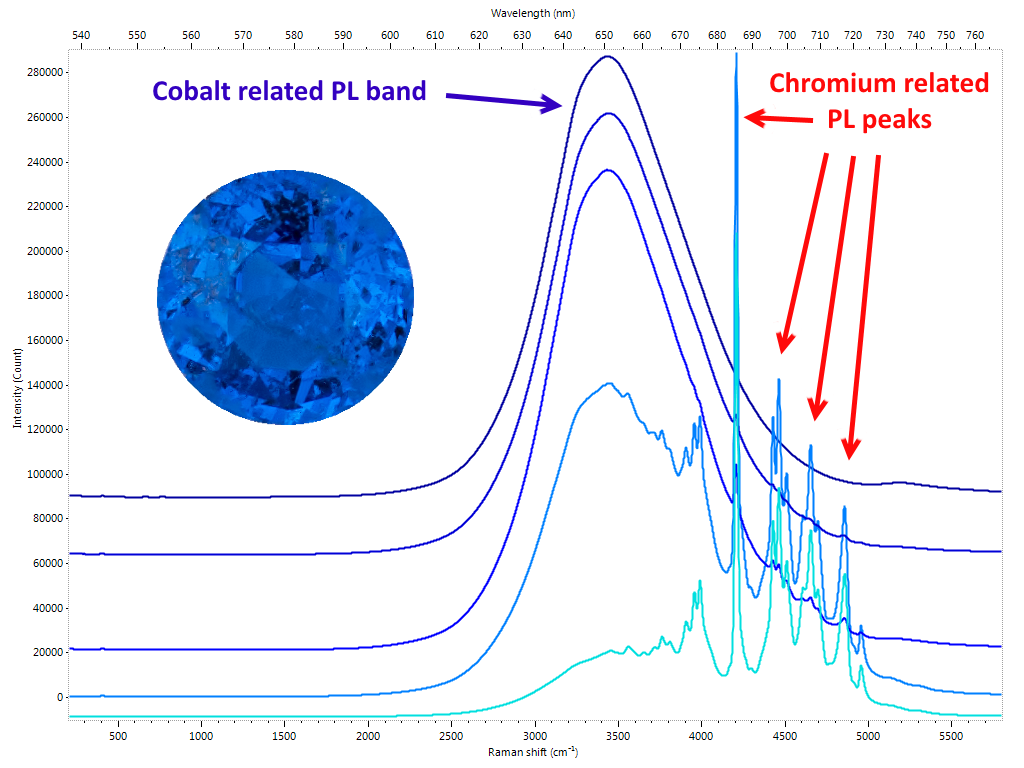
We have already highlighted how this technique has become part of the permanent endowment of the gemological laboratory to respond to a precise need for the identification of the HPHT treated diamonds. As was foreseeable, in a short time it went much further. Diamond presents a virtually limitless range of specific emissions arranged in a vast area ranging from ultraviolet to infrared. An immense field has therefore opened up for the work of gemologists: identifying, characterizing and explaining all these elements and correlating them with the enormous vastness of the types of diamonds (natural and synthetic) and related treatments. It is obvious that in order to be able only to consider dealing with a similar amount of work, it is not only necessary to equip oneself with adequate instruments, it is also necessary to have a number of samples with “certain” characteristics to compare the measurements and validate the studies. Similarly to what happens in the case of color gems, where it is essential to find – when there are requirements of geographical origin attribution – samples of “sure” provenance, in the field of diamonds it is sometimes not enough to rely on characteristics provided by the company that perform a specific treatment to be sure to have adequate material. It is not infrequent that the major laboratories and scholars in this field are “forced” to carry out by their own or to outsource the aforementioned treatments to make sure they have a consistent database. Considering cost of diamonds and of the usually expensive procedures, it is understandable how much this research activity is expensive and, sometimes, due to the scarcity of material (eg Fancy color) almost prohibitive. Despite all the research goes on expediently and a large part of the problems related to the diamond has been solved, albeit in a not yet definitive way, due to a safe and reliable investigation path by the use of PL spectroscopy. The results of these studies are periodically presented to the gemological community by means of articles and treatises. In many cases, however, the most “sensitive” data are not publicly released in order to avoid countermeasures by those who carry out the treatments to harm the market. Certainties, in gemology, are rare. Nature often creates bizarre combinations for which it is not excluded that a specific feature found only in treated or synthetic material, in rare cases may also occur in untreated/natural stones. If the identification of the NV0 and NV- PL defects and their ratio were a first effective indication of GE-POL HPHT treated type IIa diamonds, it was found very soon that this rule had exceptions, especially for almost completely colorless samples. Then there are the cases in which more types of treatments are performed in sequence, something happening more and more frequently nowadays. One of the areas in which the phenomenon is most widespread is that of fancy color diamonds. By carefully alternating HPHT treatment, irradiation and annealing, you can literally transform a frog – brown/yellowish Ia type diamond – in a beautiful princess – an outstanding pink diamond. In this case the task of the gemologist is definitely more complex: a sequence of treatments must be identified starting exclusively from the characteristics of the material that can be analyzed only at its last stage. PL spectrometry is not the only technique used, but it is certainly the one that provides the most effective clues. Other gems One of the elements most reactive to light radiation is undoubtedly chromium. Fortunately for us it is also one of the most present as trace element in gems. Rubies, emeralds, spinels, the list is very long, in many cases the presence of this element gives the stones their extraordinary beauty. Imperial topaz, tsavorite and chrome tourmaline are just some more examples . Even infinitesimal chromium impurities in the crystalline lattice of a gem are enough to generate a PL reaction which, by featuring one or more specific emission peaks, allows the gemologist to identify it. In the case of the Ruby we are talking about the doublet at 692.8 and 694.2 nm also observable through a common optical spectroscope. Although the traces of chromium in corundum are sometimes not sufficient to influence the color, its presence even in very small quantities is always enough to generate its characteristic PL spectrum (Figure 3).
In spinel, position and width of the peaks provide for further and very useful information. If, in fact, a dominant peak centered at 685.5 nm and successive bands with longer wavelengths are practically appreciable in the natural non-heated spinel, in the case spinel is synthetic or natural subjected to heat treatment, the position of the peaks will undergo a shift towards longer wavelengths and an increase in the width (Figure 4).
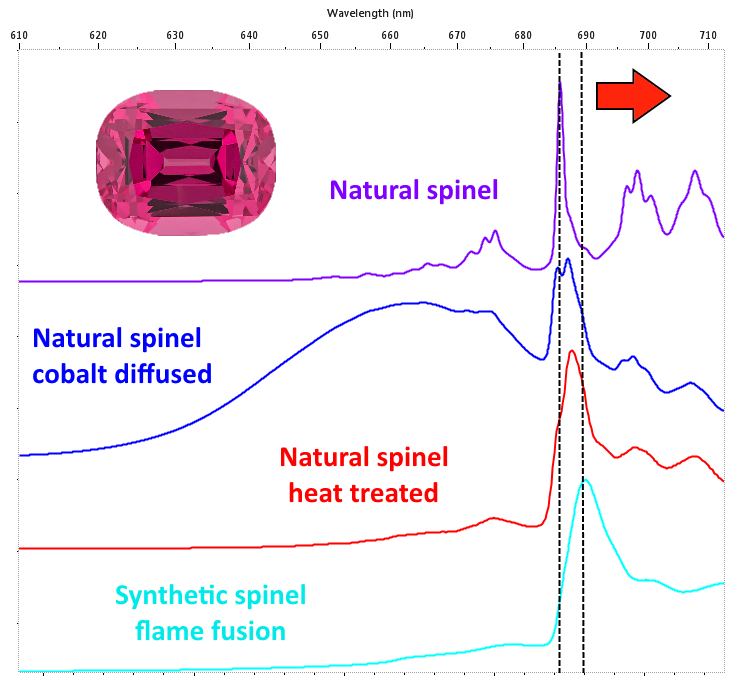
The cause is to be found in the increase in disorder in the crystal structure generated by temperature. Here is how the photoluminescence spectrum is a very effective method for identifying synthetic spinel and its heat treatment in the case of natural. Natural spinel with cobalt impurities are having lately an unprecedented commercial success. Vietnamese Luc Yen’s most prized gems exhibit a highly saturated electric blue color. The production is characterized by rarely large roughs and this means that even relatively small crystals are traded on the market at truly considerable figures. In this case too, PL spectroscopy provides us with a diagnostic test: the presence of cobalt traces in spinel can be, in fact, highlighted as a broad band centered at 650 nm (Figure 5).
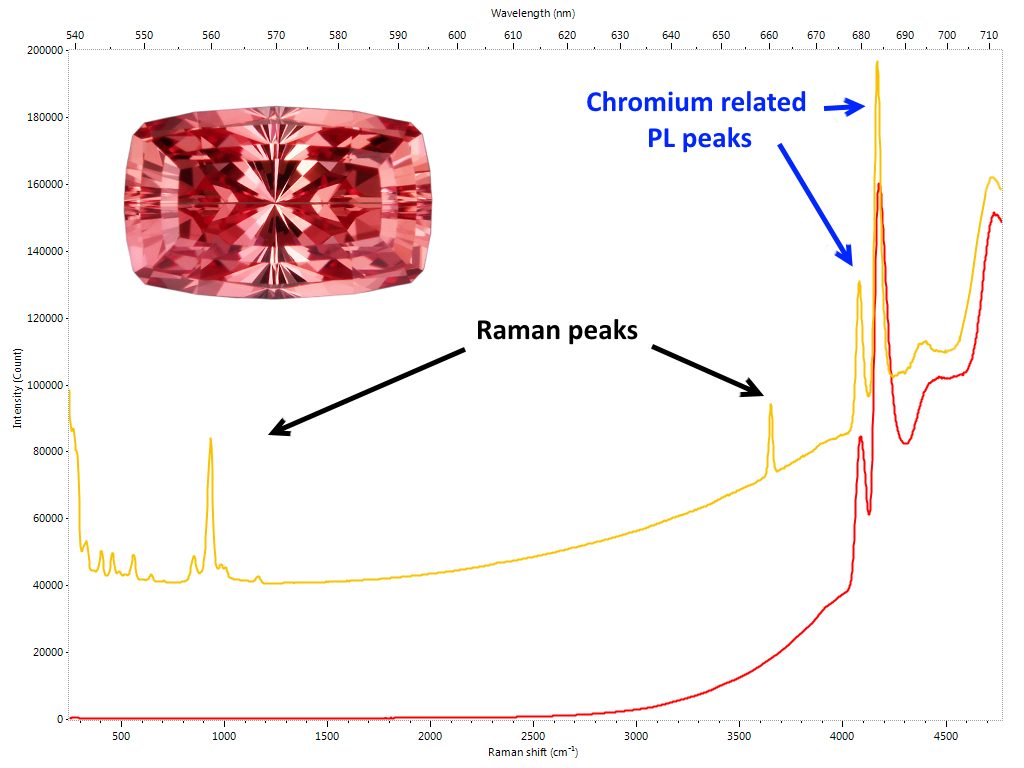
The emerald also has a PL spectrum characterized by two emission peaks due to chromium, centered respectively at 680.6 683.6 nm. Recent studies have shown that the position of these peaks is related to the geological origin of the material. More precisely, depending on whether emeralds are associated to schist or non-schist occurrence, the position of the two peaks varies slightly but in a congruous manner. It has also been possible to hypothesize a direct correlation between chromium and iron percentages present in the emerald and the intensity of the PL bands. The presence of chromium in topaz can generate a range of colors from red to pink to violet / purple. The market uses the term “imperial” associated with topaz when it has tones and saturations of the aforementioned colors that make the gem particularly attractive. Topaz is not particularly rare, however, when one can boast of the adjective of “imperial”, its value grows considerably. Consequently it is not surprising that gems of poor colors are often subjected to irradiation cycles to improve their tone and saturation in order to make them look like the best quality material. The presence on the market of these irradiated gems passed off as “imperial” is now a constant, also due to the fact that the treatment is practically not identifiable. Moreover, and this is the most dramatic aspect, irradiation acts on the topaz color centers that are often unstable. The result is that color tends to fade over time due to the temporary effect the irradiation causes on the color centers. The PL spectrum of an “imperial” topaz shows an emission due to the presence of the chromium such that, if recorded by a Raman / PL spectrometer, it does not allow to visualize the Raman spectrum in the same scan. On the other hand, a Topaz with minimal or no chromium impurities can exhibit both the Raman spectrum and the minimum possible emissions due to chromium in the same scan (Figure 6). It is intuitive that if this occurs for a gem that appears sufficiently saturated to be visually identified as “imperial”, the cause of its appearance its very unlikely to be correlated with chromium. Consequently, reasoning by exclusion, it is possible to deduce that the origin of the color is, almost certainly, an artificial treatment. Irradiation is the best guess.
Conclusions
Photoluminescence spectroscopy is considered today one of the most effective and promising techniques in the gemological investigation. Its application in the field of diamonds has proved to be of vital importance for the identification of numerous treatments and for the classification of natural material, especially for fancy-colored diamonds. Numerous gemological applications related to color gems are already regularly and permanently part of analysis protocols in gemological laboratories. In some cases, the effectiveness of PL spectroscopy allows the solution of problems that are otherwise decidedly complex to be dealt with by alternative methodologies. Being a relatively recent technique it is more than probable, indeed almost certain, that the development of new studies will considerably contribute to cope with the increasing number of critical issues that the gemologist is facing nowadays.
Bibliography
Spectroscopic evidence of GE-POL HPHT-treated natural type IIa diamonds – By David Fisher and Raymond A. Spits – Gems & Gemology, Vol XXXVI, Spring 2000.
Luminescence spectroscopy and microscopy applied to study gem materials: a case study of C centre containing diamonds – Thomas Hainschwang, Stefanos Karampelas, Emmanuel Fritsch and Franck Notari – An introduction to photoluminescence spectroscopy for diamond and its applications in gemology – Sally Eaton-Magaña and Christopher M. Breeding – Gems & Gemology, Vol LII, Spring 2016.
Photoluminescence spectra of emeralds from Colombia, Afghanistan, and Zambia – D. Brian Thompson, Christian J. Bayens, Matthew B. Morgan, Taylor J. Myrick, and Nealey E. Sims – Gems & Gemology, Vol LIII, Fall 2017.
Request your copy of Rivista Italiana di Gemmologia or buy a yearly subscription
Article by Alberto Scarani and Mikko Åström, published on Rivista Italiana di Gemmologia #3, January 2018.
















